Copper-catalyzed synthesis of gem-difluoroallylboronates from α-trifluoromethyl alkenes and B2pin2 - ScienceDirect

Reaction scheme of methane borylation with B2pin2 The molecular sizes... | Download Scientific Diagram

Enantioselective Copper-Catalyzed sp2/sp3 Diborylation of 1-Chloro-1-Trifluoromethylalkenes | ACS Central Science

Metal-Free Reduction of Aromatic Nitro Compounds to Aromatic Amines with B2pin2 in Isopropanol | Organic Letters

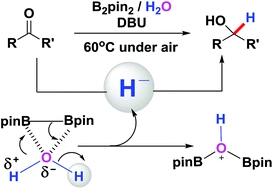
Reductive Aromatization of Quinols with B2pin2 as Deoxidizing Agent - Liu - 2020 - Chemistry – An Asian Journal - Wiley Online Library

Copper/B2pin2-catalyzed C-H difluoroacetylation-cycloamidation of anilines leading to the formation of 3,3-difluoro-2-oxindoles. | Semantic Scholar

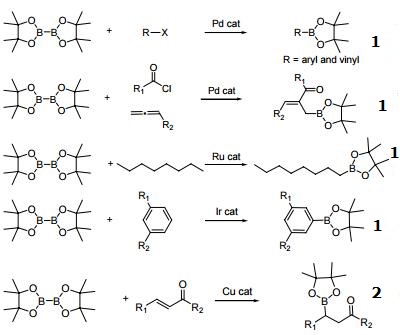
Palladium-catalyzed regioselective hydroboration of aryl alkenes with B2pin2 - Chemical Communications (RSC Publishing)

B–B bond activation and NHC ring-expansion reactions of diboron(4) compounds, and accurate molecular structures of B2(NMe2)4, B2eg2, B2neop2 and B2pin2 - Dalton Transactions (RSC Publishing)
![B2pin2-catalyzed oxidative cleavage of a C [[double bond, length as m-dash]] C double bond with molecular oxygen - Organic Chemistry Frontiers (RSC Publishing) B2pin2-catalyzed oxidative cleavage of a C [[double bond, length as m-dash]] C double bond with molecular oxygen - Organic Chemistry Frontiers (RSC Publishing)](https://pubs.rsc.org/en/Content/Image/GA/C8QO01412D)